ENZYMES INCREASE RATES OF REACTION
● `color{violet}("Catalysed reactions")` proceed at rates vastly higher than that of `color{violet}("uncatalysed ones.")`
● When `color{violet}("enzyme catalyzed reactions")` are observed, the rate would be vastly higher than the same but `color{violet}("uncatalysed reaction.")`
● For example
`underset("carbondioxide ")(CO_2) + underset("water")(H_2O) overset("carbonic anhydrase")→ underset(" carbonic acid
")(H_2CO_3)`
● In the absence of any `color{violet}("enzyme this reaction")` is `color{brown}("very slow,")` with about 200 molecules of `H_2CO_3` being formed in an hour.
● However, by using the `color{violet}("enzyme")` present within the cytoplasm called `color{brown}("carbonic anhydrase")`, the reaction speeds dramatically with about `color{violet}("600,000 molecules")` being formed every second.
● The `color{violet}("enzyme")` has accelerated the reaction rate by about `color{violet}("10 million times.")`
● There are thousands of types of enzymes each `color{violet}("catalysing a unique chemical")` or `color{violet}("metabolic reaction.")`
● A `color{violet}("multistep chemical reaction")`, when each of the steps is catalysed by the same `color{violet}("enzyme complex")` or different enzymes, is called a `color{brown}("metabolic pathway.")`
● For example, `color{violet}("Glucose →2 Pyruvic acid")`
● `color{violet}(C_6H_(12)O_6 + O_2 →2C_3H_4 O_3 + 2H_2O)` is actually a `color{violet}("metabolic pathway")` in which glucose becomes `color{violet}("pyruvic acid")` through ten different enzyme catalysed `color{violet}("metabolic reactions")`.
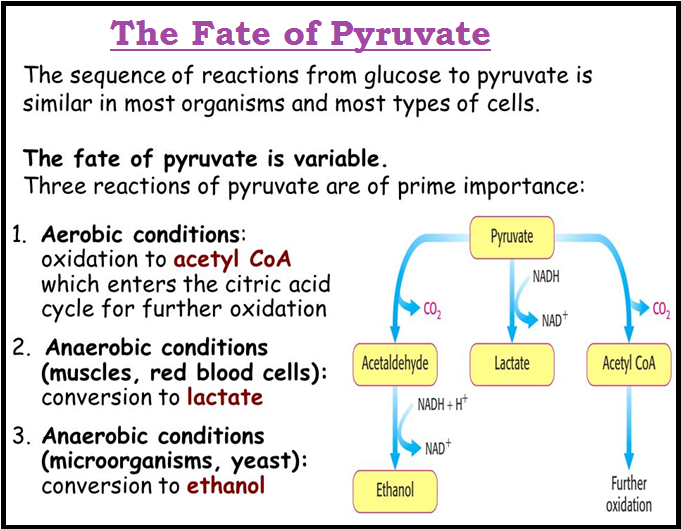
● At this stage you should know that this very `color{violet}("metabolic pathway")` with one or two additional reactions gives rise to a variety of `color{violet}("metabolic end products.")`
● In our skeletal muscle, under anaerobic conditions, `color{brown}("lactic acid")` is formed.
● Under normal aerobic conditions, `color{brown}("pyruvic acid")` is formed.
● In yeast, during `color{brown}("fermentation,")` the same pathway leads to the `color{violet}("production of ethanol (alcohol).")`
● Hence, in different conditions different products are possible.
● When `color{violet}("enzyme catalyzed reactions")` are observed, the rate would be vastly higher than the same but `color{violet}("uncatalysed reaction.")`
● For example
`underset("carbondioxide ")(CO_2) + underset("water")(H_2O) overset("carbonic anhydrase")→ underset(" carbonic acid
")(H_2CO_3)`
● In the absence of any `color{violet}("enzyme this reaction")` is `color{brown}("very slow,")` with about 200 molecules of `H_2CO_3` being formed in an hour.
● However, by using the `color{violet}("enzyme")` present within the cytoplasm called `color{brown}("carbonic anhydrase")`, the reaction speeds dramatically with about `color{violet}("600,000 molecules")` being formed every second.
● The `color{violet}("enzyme")` has accelerated the reaction rate by about `color{violet}("10 million times.")`
● There are thousands of types of enzymes each `color{violet}("catalysing a unique chemical")` or `color{violet}("metabolic reaction.")`
● A `color{violet}("multistep chemical reaction")`, when each of the steps is catalysed by the same `color{violet}("enzyme complex")` or different enzymes, is called a `color{brown}("metabolic pathway.")`
● For example, `color{violet}("Glucose →2 Pyruvic acid")`
● `color{violet}(C_6H_(12)O_6 + O_2 →2C_3H_4 O_3 + 2H_2O)` is actually a `color{violet}("metabolic pathway")` in which glucose becomes `color{violet}("pyruvic acid")` through ten different enzyme catalysed `color{violet}("metabolic reactions")`.
● At this stage you should know that this very `color{violet}("metabolic pathway")` with one or two additional reactions gives rise to a variety of `color{violet}("metabolic end products.")`
● In our skeletal muscle, under anaerobic conditions, `color{brown}("lactic acid")` is formed.
● Under normal aerobic conditions, `color{brown}("pyruvic acid")` is formed.
● In yeast, during `color{brown}("fermentation,")` the same pathway leads to the `color{violet}("production of ethanol (alcohol).")`
● Hence, in different conditions different products are possible.